
Fabry
X-linked disease caused by a mutation of the GLA gene that results in lysosomal α–galactosidase enzyme deficiency, leading to globotriaosylceramide (GL3) and other glycosphingolipids buildup.

Gaucher
Is a rare genetic disorder caused by a deficiency of the enzyme glucocerebrosidase, which is responsible for breaking down glucocerebroside.

ASMD
Acid sphingomyelinase deficiency (ASMD), also known as Niemann-Pick disease type A and type B, is a rare genetic disorder caused by a deficiency of the enzyme acid sphingomyelinase (ASM).
Manage all your sample requests and results in one place!
Program Added Value
Timely support and attention to your inquiries and results.
Visibility and tracking of your sample.

On-hand information available about the program:
diseases, gene panels, sample submission, process, results.
Partnership
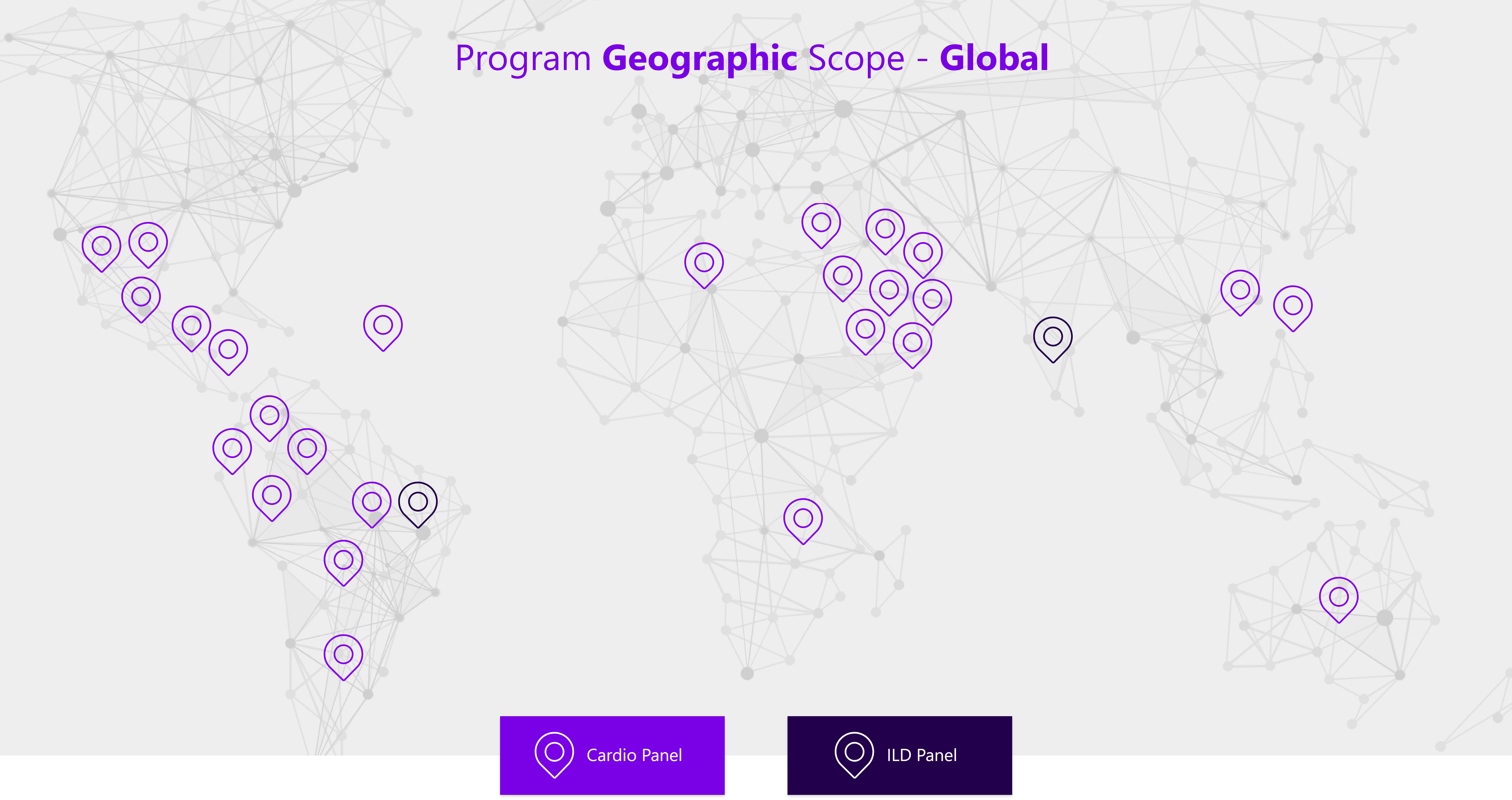
Sanofi works hand in hand with selected diagnostic laboratories and with TBTB Global
to process the samples and manage the program.


Frequently Asked Questions
-
RDGenePP is a global diagnostic program designed to aid in the diagnosis of lysosomal storage diseases. It encompasses various processes,
including the creation and distribution of diagnostic kits, sample handling and transportation, and diagnostic testing.Healthcare Professionals (HCPs), including medical practitioners, can participate in the RDGenePP to diagnose patients with suspected
lysosomal storage diseases.To access the RDGenePP and its resources, you can typically do so through your Sanofi Local Affiliates colleagues.
Diagnostic kits contain materials necessary for collecting and transporting samples for diagnostic testing. These kits are provided to HCPs
and local reference centers. They help ensure that samples are collected and transported correctly, along with proper documentation, to
facilitate accurate and reliable diagnostic testing. Each kit typically includes collection forms, test request forms, informed consent forms,
envelopes, silica gel bags, and, if applicable, educational materials. -
Informed Consent Forms ensure that patients understand the purpose and risks of diagnostic testing and voluntarily participate in the
program. They are essential for ethical and legal reasons and must be signed so the laboratory can proceed with the sample analysis.Samples are collected using the Dry Blood Sample (DBS) method, which involves a minimally invasive process. A small volume of blood is
obtained from the patient’s fingertip and placed on a special filter paper. The DBS is then dried and stored until it is ready to be shipped to
the diagnostic laboratory.After collecting and preparing the sample, you will follow specific guidelines for packaging it in the provided diagnostic kit. The kit includes
essential forms and materials. Once the sample is ready, it can be sent to the local Reference Center or directly to the diagnostic laboratory,
through designated international logistics vendor. -
The turnaround time (TAT) to receive diagnostics results may vary but is typically within 30 business days after the arrival of the sample in the
laboratory (NGS pannel results TAT is 20 business days and enzymatic results TAT is 5-8 business days).Yes, the program provides tracking mechanisms and tools. You can use the RDGenePP App to monitor the status of your samples and
access the results when they are ready.HCPs can access and view diagnostic results through the program’s digital platform, RDGenePP App which provides secure access to
patient-specific results while ensuring compliance with data privacy regulations. Once results are ready to view, you will receive a push
notification and an email from: rdgenepp@tbtbglobal.comIf you encounter any issues or have questions while participating in the RDGenePP, you can seek support or through the messages section in
your RDGenePP App.Patient confidentiality and data privacy are paramount in the RDGenePP. The program complies with all applicable laws and regulations to
safeguard patient information and maintain strict confidentiality.
Let us help you with any questions!
Do not hesitate on contacting us!
